TWENTY-SECOND ISSUE
NOVEMBER 16, 2022
Risk Factors for Vancomycin Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Syndrome
JAMA Dermatology
DRESS is more: beware the risks of high vancomycin dosing!
Vancomycin is an antibiotic utilized to treat infections caused by gram positive organisms, particularly bacteremia, endocarditis, and osteomyelitis due to MRSA. IV vancomycin is associated with severe adverse reactions, including Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Vancomycin-induced DRESS is associated with higher fatality rates than DRESS caused by other medications.
This case-control study compared patients administered IV vancomycin who developed DRESS following >1 week of treatment (54 patients) and those who received IV vancomycin for 1-8 weeks without DRESS (1302 patients). The study utilized logistic regression with outcomes expressed as adjusted odds ratios to assess for risk factors associated with IV vancomycin-induced DRESS.
Risk of developing DRESS due to IV vancomycin was increased in patients under the age of 50 (aOR 3.21; 95% CI, 1.33-7.78). Risk was also increased with a vancomycin trough of more than 25 μg/mL and 30 μg/mL (aOR, 2.32 [95% CI, 1.18-5.11] and 8.69 [95% CI, 3.39-22.27], respectively). Patients with hypertension had a decreased risk for DRESS (aOR, 0.45; 95% CI, 0.25-0.82). Sex, race, BMI, comorbidities, and presence of other allergies were not associated with an increased risk of DRESS.
Limitations: Vancomycin-tolerant patients were chosen from an outpatient center administering parenteral medications, which may have led to selection bias.
Main Takeaways: Avoidance of DRESS in patients prescribed IV vancomycin requires correct dosing adjustments (think renal dosing) and trough monitoring, especially in patients younger than 50 years old.
Vancomycin is an antibiotic utilized to treat infections caused by gram positive organisms, particularly bacteremia, endocarditis, and osteomyelitis due to MRSA. IV vancomycin is associated with severe adverse reactions, including Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS). Vancomycin-induced DRESS is associated with higher fatality rates than DRESS caused by other medications.
This case-control study compared patients administered IV vancomycin who developed DRESS following >1 week of treatment (54 patients) and those who received IV vancomycin for 1-8 weeks without DRESS (1302 patients). The study utilized logistic regression with outcomes expressed as adjusted odds ratios to assess for risk factors associated with IV vancomycin-induced DRESS.
Risk of developing DRESS due to IV vancomycin was increased in patients under the age of 50 (aOR 3.21; 95% CI, 1.33-7.78). Risk was also increased with a vancomycin trough of more than 25 μg/mL and 30 μg/mL (aOR, 2.32 [95% CI, 1.18-5.11] and 8.69 [95% CI, 3.39-22.27], respectively). Patients with hypertension had a decreased risk for DRESS (aOR, 0.45; 95% CI, 0.25-0.82). Sex, race, BMI, comorbidities, and presence of other allergies were not associated with an increased risk of DRESS.
Limitations: Vancomycin-tolerant patients were chosen from an outpatient center administering parenteral medications, which may have led to selection bias.
Main Takeaways: Avoidance of DRESS in patients prescribed IV vancomycin requires correct dosing adjustments (think renal dosing) and trough monitoring, especially in patients younger than 50 years old.
A review of adverse ocular events associated with topical corticosteroid use
Journal of The American Academy of Dermatology
Bringing steroid side effects into focus!
While topical corticosteroids (TCS) are widely used in dermatology for a variety of skin pathologies and have well-demonstrated efficacy; they also pose risks for side effects. Prolonged use of systemic corticosteroid therapy can cause serious ocular complications such as glaucoma and cataracts, but the association between TCS use and ocular events is less clear.
This systematic review analyzed all reported cases of cataracts and glaucoma associated with TCS use. Researchers searched MEDLINE for relevant keywords, and two investigators independently assessed reports (ophthalmic and intranasal TCS administration were excluded). Fifty unique patients who met inclusion criteria were identified. Analysis showed that patients were on average 38 years old, and 62% were male. 60% of patients assessed had only glaucoma, 14% had only cataracts, and 26% had both. TCS strength varied among patients; 18% used only low-potency TCS (e.g. hydrocortisone 2.5% cream), 36% used medium or high-potency TCS (e.g. triamcinolone 0.1% ointment or stronger), and 46% had unknown TCS type. 78% of patients reported facial application of TCS, while 54% of these reported periorbital use. The mean duration of treatment was 8 years. Importantly, 78% of patients had atopic dermatitis, a known risk factor for cataract formation, which could represent a confounding bias. Additionally, 33% had a personal or family history of ocular disease.
Limitations: Small sample size and inclusion of low-quality case reports/series.
Main Takeaway: There is a paucity of high-quality evidence that supports a causal relationship between topical corticosteroid use and adverse ocular events. Proper TCS use is unlikely to be associated with cataract and glaucoma formation.
While topical corticosteroids (TCS) are widely used in dermatology for a variety of skin pathologies and have well-demonstrated efficacy; they also pose risks for side effects. Prolonged use of systemic corticosteroid therapy can cause serious ocular complications such as glaucoma and cataracts, but the association between TCS use and ocular events is less clear.
This systematic review analyzed all reported cases of cataracts and glaucoma associated with TCS use. Researchers searched MEDLINE for relevant keywords, and two investigators independently assessed reports (ophthalmic and intranasal TCS administration were excluded). Fifty unique patients who met inclusion criteria were identified. Analysis showed that patients were on average 38 years old, and 62% were male. 60% of patients assessed had only glaucoma, 14% had only cataracts, and 26% had both. TCS strength varied among patients; 18% used only low-potency TCS (e.g. hydrocortisone 2.5% cream), 36% used medium or high-potency TCS (e.g. triamcinolone 0.1% ointment or stronger), and 46% had unknown TCS type. 78% of patients reported facial application of TCS, while 54% of these reported periorbital use. The mean duration of treatment was 8 years. Importantly, 78% of patients had atopic dermatitis, a known risk factor for cataract formation, which could represent a confounding bias. Additionally, 33% had a personal or family history of ocular disease.
Limitations: Small sample size and inclusion of low-quality case reports/series.
Main Takeaway: There is a paucity of high-quality evidence that supports a causal relationship between topical corticosteroid use and adverse ocular events. Proper TCS use is unlikely to be associated with cataract and glaucoma formation.
How does vitamin D supplementation during pregnancy affect infantile eczema?
British Journal of Dermatology
Antenatal Vitamin D: it’s all sun and games!
It is now thought that infantile eczema arises in part in utero and can be affected by environmental exposures on fetal and immunologic development. Observationally, several studies suggest that the risk of eczema in a child may be linked to antenatal vitamin D supplementation (although in inconsistent directions). Until now, no prior study had specifically tested the hypothesis that vitamin D supplementation during pregnancy could decrease the risk of infantile eczema. Thus, the authors conducted the UK Maternal Vitamin D Osteoporosis Study, which was a double-blind, randomized, placebo-controlled trial. Pregnant patients either received 1000IU vitamin D or placebo from 14 weeks gestation to delivery, and the prevalence of eczema in each group was measured at 12 months, 24 months, and 48 months of age. The authors found that vitamin D supplementation was associated with a significantly decreased risk of atopic eczema at 12 months (p=0.04) and trended towards significance at 24 months and 48 months. They postulated that the mechanism may be via increased breast milk cholecalciferol levels.
Main Takeaway: In this study, antenatal vitamin D supplementation is associated with a decreased risk of infantile atopic eczema.
It is now thought that infantile eczema arises in part in utero and can be affected by environmental exposures on fetal and immunologic development. Observationally, several studies suggest that the risk of eczema in a child may be linked to antenatal vitamin D supplementation (although in inconsistent directions). Until now, no prior study had specifically tested the hypothesis that vitamin D supplementation during pregnancy could decrease the risk of infantile eczema. Thus, the authors conducted the UK Maternal Vitamin D Osteoporosis Study, which was a double-blind, randomized, placebo-controlled trial. Pregnant patients either received 1000IU vitamin D or placebo from 14 weeks gestation to delivery, and the prevalence of eczema in each group was measured at 12 months, 24 months, and 48 months of age. The authors found that vitamin D supplementation was associated with a significantly decreased risk of atopic eczema at 12 months (p=0.04) and trended towards significance at 24 months and 48 months. They postulated that the mechanism may be via increased breast milk cholecalciferol levels.
Main Takeaway: In this study, antenatal vitamin D supplementation is associated with a decreased risk of infantile atopic eczema.
How does intralesional purified protein derivative compare to intralesional zinc sulfate 2% in the treatment of pediatric warts?
Journal of Pediatric Dermatology
Warts in a ~nutshell~: treat them with purified protein derivative and zinc sulfate!
Warts are small, benign growths caused by a human papilloma virus (HPV) infection of the skin or mucous membrane. They can affect up to 30% of primary school children. There is currently no specific treatment against HPV infection, and warts can be difficult to treat. Current treatment options include epidermal destruction or keratolysis, immunotherapy, and cytotoxic agents. In addition, intralesional purified protein derivative (PPD) and zinc sulfate 2% have been used in the treatment of warts. However, few studies have compared the efficacy, safety, and tolerability of PPD compared to zinc sulfate 2% in the treatment of pediatric warts.
The researchers of this study conducted a randomized clinical trial of 120 patients between ages 4 and 18 with multiple warts. Patients were randomly divided into two treatment groups: 1) intralesional PPD at a dose of 10 IU, or 2) intralesional zinc sulfate 2%. The appropriate treatment was injected into the largest wart every 2 weeks until clearance or a maximum of 5 treatment sessions was reached. Clinical efficacy was categorized as complete (100% reduction in wart size), partial (50-99% reduction), or poor (0-50% reduction).
The overall response to all warts was equal in both the PPD and zinc sulfate group (81.7%), but the response of the injected wart was higher in the zinc sulfate group (93.4%) compared to the PPD group (83.3%). The zinc sulfate group had a slightly lower median number of sessions to achieve complete cure compared to PPD (3 vs 4 sessions). Highest cure rates were after the 1st session in the zinc sulfate group and 5th session in the PPD group. Of note, adverse events were significantly higher in the zinc sulfate 2% group, including pain (70%), inflammation (26.7%), ulceration (8.3%), necrosis (8.3%), and scars (16.7%). The only significant adverse event for PPD was injection site itching (13.3%).
Limitations: Further studies are needed to investigate whether efficacy is improved with injection of higher doses and multiple warts. Future studies to determine the ideal interval between injections are also needed.
Main Takeaway: This study found that both intralesional PPD and zinc sulfate 2% are effective in pediatric warts, although PPD had a higher safety profile.
Warts are small, benign growths caused by a human papilloma virus (HPV) infection of the skin or mucous membrane. They can affect up to 30% of primary school children. There is currently no specific treatment against HPV infection, and warts can be difficult to treat. Current treatment options include epidermal destruction or keratolysis, immunotherapy, and cytotoxic agents. In addition, intralesional purified protein derivative (PPD) and zinc sulfate 2% have been used in the treatment of warts. However, few studies have compared the efficacy, safety, and tolerability of PPD compared to zinc sulfate 2% in the treatment of pediatric warts.
The researchers of this study conducted a randomized clinical trial of 120 patients between ages 4 and 18 with multiple warts. Patients were randomly divided into two treatment groups: 1) intralesional PPD at a dose of 10 IU, or 2) intralesional zinc sulfate 2%. The appropriate treatment was injected into the largest wart every 2 weeks until clearance or a maximum of 5 treatment sessions was reached. Clinical efficacy was categorized as complete (100% reduction in wart size), partial (50-99% reduction), or poor (0-50% reduction).
The overall response to all warts was equal in both the PPD and zinc sulfate group (81.7%), but the response of the injected wart was higher in the zinc sulfate group (93.4%) compared to the PPD group (83.3%). The zinc sulfate group had a slightly lower median number of sessions to achieve complete cure compared to PPD (3 vs 4 sessions). Highest cure rates were after the 1st session in the zinc sulfate group and 5th session in the PPD group. Of note, adverse events were significantly higher in the zinc sulfate 2% group, including pain (70%), inflammation (26.7%), ulceration (8.3%), necrosis (8.3%), and scars (16.7%). The only significant adverse event for PPD was injection site itching (13.3%).
Limitations: Further studies are needed to investigate whether efficacy is improved with injection of higher doses and multiple warts. Future studies to determine the ideal interval between injections are also needed.
Main Takeaway: This study found that both intralesional PPD and zinc sulfate 2% are effective in pediatric warts, although PPD had a higher safety profile.
innovations in dermatology
‘UV’ heard of 3D printed, flexible photodetectors?
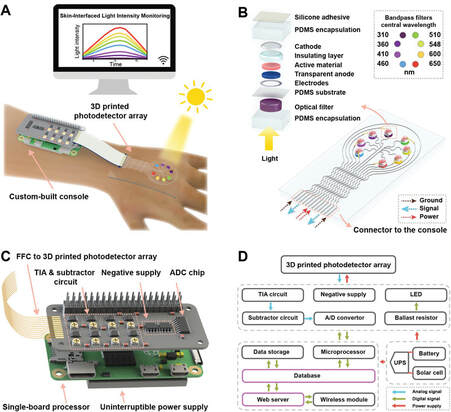
Lupus erythematosus (LE), amongst other skin diseases, are significantly aggravated by Ultraviolet (UV) exposure and even ambient indoor light (yes, we’re talking about the electromagnetic spectrum!). But, what if these patients could wear a device that alerted them to the exposure level that exacerbates their disease? Currently, there are commercially available silicon photodetectors, but these devices may have difficulty adhering to the skin and lack flexibility in detecting absorption over a broadband spectrum of both UV and visible light.
To advance this technology, researchers at the University of Minnesota incorporated organic semiconductors to enhance the photoactive material and mechanical flexibility of the detector. Amplification of the signals to the photodetector was accomplished by the introduction of certain photoreceptive molecules, such as Zinc Oxide (ZnO), which effectively widened the spectral range of detection. 3D printing was utilized to incorporate the nanoparticles into the material to create a flexible, skin-interfaced photodetector array in accordance with a custom-coded Python based Wi-Fi system to interface the constant web browser monitoring system. The hybrid nanoparticle material was found to trigger the photo multiplication effect and improve the photodetector response across all UV bands. The device can be utilized with a simple lithium rechargeable battery allowing for all day wear without worry.
During testing it processed the ability to monitor outdoor broadband light intensity for nearly 24h. The 3D printed photodetector is able to continuously monitor and record the long-term light intensity distributions through its UV-vis photodetector allowing for real time exposure monitoring for LE patients to determine broadband effects. The future studies intend to extend the photoresponse to the near infrared region as well as targeting photoprotection for other photosensitive diseases to broaden the applications of the device and miniaturizing the system to increase usability.
Main takeaway: The 3D printed photodetector is able to continuously monitor and record long-term light intensity distributions allowing for real time monitoring for LE patients and has future applicability to other photosensitive diseases.
Lupus erythematosus (LE), amongst other skin diseases, are significantly aggravated by Ultraviolet (UV) exposure and even ambient indoor light (yes, we’re talking about the electromagnetic spectrum!). But, what if these patients could wear a device that alerted them to the exposure level that exacerbates their disease? Currently, there are commercially available silicon photodetectors, but these devices may have difficulty adhering to the skin and lack flexibility in detecting absorption over a broadband spectrum of both UV and visible light.
To advance this technology, researchers at the University of Minnesota incorporated organic semiconductors to enhance the photoactive material and mechanical flexibility of the detector. Amplification of the signals to the photodetector was accomplished by the introduction of certain photoreceptive molecules, such as Zinc Oxide (ZnO), which effectively widened the spectral range of detection. 3D printing was utilized to incorporate the nanoparticles into the material to create a flexible, skin-interfaced photodetector array in accordance with a custom-coded Python based Wi-Fi system to interface the constant web browser monitoring system. The hybrid nanoparticle material was found to trigger the photo multiplication effect and improve the photodetector response across all UV bands. The device can be utilized with a simple lithium rechargeable battery allowing for all day wear without worry.
During testing it processed the ability to monitor outdoor broadband light intensity for nearly 24h. The 3D printed photodetector is able to continuously monitor and record the long-term light intensity distributions through its UV-vis photodetector allowing for real time exposure monitoring for LE patients to determine broadband effects. The future studies intend to extend the photoresponse to the near infrared region as well as targeting photoprotection for other photosensitive diseases to broaden the applications of the device and miniaturizing the system to increase usability.
Main takeaway: The 3D printed photodetector is able to continuously monitor and record long-term light intensity distributions allowing for real time monitoring for LE patients and has future applicability to other photosensitive diseases.