Fifty-eighth issue
April 17, 2024
Do patients who received rituximab as first-line therapy for pemphigus exhibit long-term remission?
JAMA Dermatology
JAMA Dermatology
Bring out your ri-TUX-imab! Pemphigus treatment has a black-tie dress code these days!
Pemphigus is a rare, autoimmune, bullous disease that classically affects the mucosa and skin. Typical treatment includes systemic corticosteroids and immunosuppressive agents such as rituximab. Authors conducted a 7-year follow-up of the Ritux 3 trial, which previously demonstrated the short-term efficacy and safety of rituximab used in combination with low-dose oral corticosteroids (LDOC). This study assessed the long-term efficacy and safety of combination treatment with rituximab and LDOCs compared with corticosteroids alone.
What did they find?
Main Takeaway: First-line treatment of pemphigus with rituximab in combination with LDOC is associated with long-term disease remission and fewer adverse events compared to corticosteroids alone.
Pemphigus is a rare, autoimmune, bullous disease that classically affects the mucosa and skin. Typical treatment includes systemic corticosteroids and immunosuppressive agents such as rituximab. Authors conducted a 7-year follow-up of the Ritux 3 trial, which previously demonstrated the short-term efficacy and safety of rituximab used in combination with low-dose oral corticosteroids (LDOC). This study assessed the long-term efficacy and safety of combination treatment with rituximab and LDOCs compared with corticosteroids alone.
What did they find?
- 43 patients from the rituximab + LDOC group (93%) and 17 patients from the corticosteroid group alone (39%) achieved complete remission
- Patients from the rituximab + LDOC group had longer 5- and 7-year disease-free survival (76.7% and 72.1% vs 35.3% and 35.3%, respectively; P < .001)
- Patients from the rituximab + LDOC group experienced roughly half the rate of disease relapse (42.2% vs 83.7%; P < .001)
- Fewer significant adverse events were reported in the rituximab + LDOC group compared to the corticosteroid only group (0.67 and 1.32 SAEs per patient respectively, P = .003)
Main Takeaway: First-line treatment of pemphigus with rituximab in combination with LDOC is associated with long-term disease remission and fewer adverse events compared to corticosteroids alone.
Efficacy and safety of crisaborole ointment, 2%, in participants aged ≥45 years with stasis dermatitis
Journal of American Academy Dermatology
Journal of American Academy Dermatology
Crisaborole cleaning up our legs one dose at a time!
Stasis dermatitis (SD) is an inflammatory skin disease that causes eczematous, scaling skin with weeping patches and plaques on the legs and feet. Current treatment options for SD include topical corticosteroids and topical calcineurin inhibitors, both of which are associated with known side effects.
This randomized, double blind, control study sought to evaluate the efficacy and safety of Crisaborole ointment 2%, a topical nonsteroidal phosphodiesterase 4 (PDE4) inhibitor.. 65 patients were randomized 1:1 to receive either crisaborole 2% or a vehicle ointment twice-daily for 6 weeks.
What did they find?
Main Takeaway: Crisaborole ointment 2% shows promise as an effective and generally well-tolerated treatment option for stasis dermatitis.
Stasis dermatitis (SD) is an inflammatory skin disease that causes eczematous, scaling skin with weeping patches and plaques on the legs and feet. Current treatment options for SD include topical corticosteroids and topical calcineurin inhibitors, both of which are associated with known side effects.
This randomized, double blind, control study sought to evaluate the efficacy and safety of Crisaborole ointment 2%, a topical nonsteroidal phosphodiesterase 4 (PDE4) inhibitor.. 65 patients were randomized 1:1 to receive either crisaborole 2% or a vehicle ointment twice-daily for 6 weeks.
What did they find?
- Percent change from baseline in total sign score (TSS) was significantly greater in patients treated with crisaborole 2% versus vehicle based on in-person assessment and central reader assessment ( -32.4% vs -18.1%, P = 0.0299 and 52.5% vs -10.3%, P = 0.0004, respectively).
- Patients treated with crisaborole 2% had a greater change from baseline in lesion % body surface area (-34.3% vs 18.7%, P = 0.0416).
- Pruritus, erythema, and contact dermatitis were the most common all-causality treatment-emergent adverse events (n = 3, 9.1%; n = 2, 6.1%; n = 2, 6.1%, respectively)
Main Takeaway: Crisaborole ointment 2% shows promise as an effective and generally well-tolerated treatment option for stasis dermatitis.
Is berdazimer gel efficacious in pediatric molluscum regardless of eczema status?
Pediatric Dermatology Journal
Pediatric Dermatology Journal
Could berdazimer be the ~counterspell~ against molluscum?
Molluscum contagiosum (MC) is a common skin infection characterized by small umbilicated lesions, primarily affecting young children with an increased prevalence in those with atopic dermatitis (AD). Traditional treatments for MC, such as cryotherapy or cantharidin application, show decreased efficacy in patients with AD.
Berdazimer gel, a topical antiviral medication which releases nitric oxide at the time of application, may be an effective and safe treatment option for MC, particularly in patients with AD. Multicenter, randomized, double-blind, vehicle-controlled, parallel-group, Phase 3 studies were conducted in 1598 pediatric patients with MC to evaluate the efficacy and safety of topical berdazimer gel 10.3%.
What did they find?
Limitation: Trials enrolled predominantly Caucasian patients from the United States, limiting generalizability.
Main Takeaways: Berdazimer gel appears efficacious in clearing molluscum contagiosum lesions in pediatric patients both with and without atopic dermatitis, while demonstrating minimal adverse events.
Molluscum contagiosum (MC) is a common skin infection characterized by small umbilicated lesions, primarily affecting young children with an increased prevalence in those with atopic dermatitis (AD). Traditional treatments for MC, such as cryotherapy or cantharidin application, show decreased efficacy in patients with AD.
Berdazimer gel, a topical antiviral medication which releases nitric oxide at the time of application, may be an effective and safe treatment option for MC, particularly in patients with AD. Multicenter, randomized, double-blind, vehicle-controlled, parallel-group, Phase 3 studies were conducted in 1598 pediatric patients with MC to evaluate the efficacy and safety of topical berdazimer gel 10.3%.
What did they find?
- At 12 weeks, berdazimer gel demonstrated comparable efficacy in achieving >90% clearance of MC lesions in patients with AD (44.5% in the berdazimer group vs 28.3% in the vehicle group; OR, 1.3; 95% CI, 1.1-3.7) and without AD (37.7% vs 22.7%; OR, 2.2; 95% CI, [1.7-2.8])
- Complete clearance rates were higher for berdazimer compared with vehicle in patients with AD (5.0% vs. 27.4%; OR, 1.3; 95% CI, [0.7–2.5]) and without AD (29.1% vs. 18.9%; OR 1.8; 95% CI, [1.4–2.4])
- Adverse events reported in patients with AD included application-site pain (21.6% with berdazimer vs 11.9% with vehicle), dermatitis (12.8% vs 2.4%), and application-site erythema (9.6% vs 7.1%)
- Application-site scarring was less frequent in the berdazimer group for patients both with and without AD (5.6% in the berdazimer group vs 10.7% in the vehicle group and 3.8% vs 6.4%, respectively)
Limitation: Trials enrolled predominantly Caucasian patients from the United States, limiting generalizability.
Main Takeaways: Berdazimer gel appears efficacious in clearing molluscum contagiosum lesions in pediatric patients both with and without atopic dermatitis, while demonstrating minimal adverse events.
Retrospective review of using seton drainage for the management of Hidradenitis suppurativa
Journal of American Academy Dermatology
Journal of American Academy Dermatology
HS goes down the seton drain!
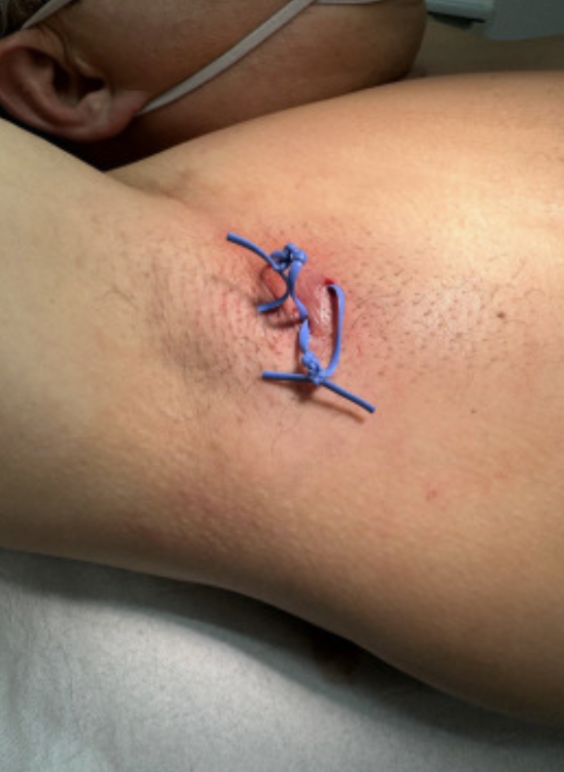
Seton drains are plastic or silicone bands that have been previously used in the management of perianal fistulas by allowing the fistulas to drain and heal internally. It has been hypothesized that use of these drains in treating hidradenitis suppurativa (HS), an inflammatory skin condition that may lead to painful tunneling of the skin with subsequent infection, could improve clinical outcomes. Placement of seton drains could allow for healing of tunnels and thus improve symptoms and outcomes. In this study, seton drains were placed in 27 patients with HS and evaluated for possible surgical intervention following removal. The Hurley staging system was utilized in characterizing the extent of the HS.
Seton drains are plastic or silicone bands that have been previously used in the management of perianal fistulas by allowing the fistulas to drain and heal internally. It has been hypothesized that use of these drains in treating hidradenitis suppurativa (HS), an inflammatory skin condition that may lead to painful tunneling of the skin with subsequent infection, could improve clinical outcomes. Placement of seton drains could allow for healing of tunnels and thus improve symptoms and outcomes. In this study, seton drains were placed in 27 patients with HS and evaluated for possible surgical intervention following removal. The Hurley staging system was utilized in characterizing the extent of the HS.
Figure 1. Seton drains placed in two different axillary tunnels.
What did they find?
Main Takeaway: The use of seton drains in the treatment of HS can help to reduce the depths of tunnels, control pain and symptoms, and enhance patient outcomes.
What did they find?
- 18 patients with Hurley II and 9 patients with Hurley III with a total of 34 tunnels were included with the axilla (47.05%) being most frequently affected
- The average seton placement duration was 5.38 weeks
- Significant (P < 0.001) reduction in average tunnel depth from 6.46 mm to 3.57 mm and significant (P < 0.001) reduction in inflammation, as seen in color doppler, were observed following removal of seton drains
Main Takeaway: The use of seton drains in the treatment of HS can help to reduce the depths of tunnels, control pain and symptoms, and enhance patient outcomes.
Drug-induced photosensitivity (DIP) occurs when a medication causes an unexpected sunburn, with the most common types being photosensitivity, photoallergic, and phototoxic reactions. Phototoxic drug reactions are defined as direct tissue damage mediated by reactive oxygen species. We present a case of a patient with severe dronedarone-induced phototoxicity.
Report of Case:
Report of Case:
- An 85-year-old female, with a past medical history of atrial fibrillation, chronic kidney disease, hypertension, and hyperlipidemia, was admitted with a three-day onset of a severe, painful, and itchy rash that developed after she had spent time swimming outdoors.
- One month prior she was prescribed dronedarone for rate control of her atrial fibrillation.
- On examination, she had crusted erosions, patches, plaques, and tense bullae on an erythematous base on her face, upper trunk, and bilateral extremities.
- A shave biopsy from the right lower extremity revealed sloughed epidermis with spongiosis, subepidermal vesiculation, scattered necrotic keratinocytes, and small foci of full-thickness epidermal necrosis with overlying parakeratosis and no significant inflammatory cell infiltrate. Direct immunofluorescence was negative.
- Given recent dronedarone initiation, the findings were most consistent with the DIP.
- Dronedarone was discontinued, and the patient was rate controlled with diltiazem. She was treated with prednisone 40 mg for five days and triamcinolone 0.1% cream. The eruption significantly improved, and she was discharged home three days later.