sixth issue
March 23, 2022
Solid Organ Transplant Recipients with Certain Risk Factors Could Benefit from Additional Surveillance for Non Keratinocyte Skin Cancers
JAMA Dermatology
Receive a transplant to cure one disease, but then end up with skin cancer after–not ideal. Solid organ transplant recipients (SOTRs) and their associated immunosuppression can be associated with increased risk for cancers, such in the skin or from certain viruses. A population-based cohort study was conducted that included 444,497 SOTRs who underwent a transplant in the US between January 1, 1987, and December 31, 2017, using linked data from the national transplant registry and 32 cancer registries. A total of 2,380 nonkeratinocyte skin cancers were identified among 444,497 SOTRs, corresponding to a 2-fold increased risk compared with the general population (SIR, 2.18; 95% CI, 2.09-2.27; P < .001) and an excess absolute risk of 50.3 additional skin cancers per 100 000 person-years. The most common skin cancer was melanoma (61.8%). The use of a mammalian target of rapamycin (mTOR) inhibitor was associated with decreased risk of melanoma (IRR, 0.75; 95% CI, 0.57-0.98) but increased risk of MCC (IRR, 1.87; 95% CI, 1.24-2.81). Other factors associated with either decreased or increased risk of certain skin cancers were age (≥65 years compared with 0-34 years) at transplant, UV radiation exposure, viruses, prolonged time since transplantation (≥10 years vs <1 year), a diagnosis of post transplant BCC or SCC, the organ transplanted, and specific immunosuppressive therapy utilized. This study suggests that SOTRs with certain risk factors should undergo more frequent surveillance for certain skin cancers.
Immune checkpoint inhibitors: what should we know about cutaneous adverse events with these cancer treatments?
Journal of American Academy of Dermatology
When the power shuts your lights off, but then you remember you have a generator! Immune checkpoint inhibitors (ICIs) work by keeping your immune system “on” (like a generator keeps the lights on!) so that it can fight off the cancer. There are different types, including CTLA-4 (e.g. ipilimumab), PD-1 (e.g. pembrolizumab) and PD-L1 (atezolizumab) inhibitors (see mechanisms below!). While ICIs have advanced the treatment of multiple cancers, including melanoma, the side effects are the real deal and can manifest in the skin. This retrospective cohort study evaluated a national claims database with 8,637 cancer patients treated with ICIs in order to better understand cutaneous immune-related adverse events. They found that approximately 25% of patients treated with ICIs developed cutaneous side effects. The most common side effects included pruritus, mucositis, erythroderma, maculopapular eruption, Grover disease, and autoimmune/inflammatory type skin diseases such as vitiligo, lichen planus, and bullous pemphigoid (makes sense since your immune system is being revved up!). Median time to onset was 113 days (3-4 months) into treatment. Limitations include the retrospective nature of this study and inability to chart review to determine dermatologic diagnoses captured by broader diagnostic codes. Regardless, this type of study can help dermatologists better understand the timing and types of side effects to expect from ICIs and better diagnose and manage these patients.
JAMA Dermatology
Receive a transplant to cure one disease, but then end up with skin cancer after–not ideal. Solid organ transplant recipients (SOTRs) and their associated immunosuppression can be associated with increased risk for cancers, such in the skin or from certain viruses. A population-based cohort study was conducted that included 444,497 SOTRs who underwent a transplant in the US between January 1, 1987, and December 31, 2017, using linked data from the national transplant registry and 32 cancer registries. A total of 2,380 nonkeratinocyte skin cancers were identified among 444,497 SOTRs, corresponding to a 2-fold increased risk compared with the general population (SIR, 2.18; 95% CI, 2.09-2.27; P < .001) and an excess absolute risk of 50.3 additional skin cancers per 100 000 person-years. The most common skin cancer was melanoma (61.8%). The use of a mammalian target of rapamycin (mTOR) inhibitor was associated with decreased risk of melanoma (IRR, 0.75; 95% CI, 0.57-0.98) but increased risk of MCC (IRR, 1.87; 95% CI, 1.24-2.81). Other factors associated with either decreased or increased risk of certain skin cancers were age (≥65 years compared with 0-34 years) at transplant, UV radiation exposure, viruses, prolonged time since transplantation (≥10 years vs <1 year), a diagnosis of post transplant BCC or SCC, the organ transplanted, and specific immunosuppressive therapy utilized. This study suggests that SOTRs with certain risk factors should undergo more frequent surveillance for certain skin cancers.
Immune checkpoint inhibitors: what should we know about cutaneous adverse events with these cancer treatments?
Journal of American Academy of Dermatology
When the power shuts your lights off, but then you remember you have a generator! Immune checkpoint inhibitors (ICIs) work by keeping your immune system “on” (like a generator keeps the lights on!) so that it can fight off the cancer. There are different types, including CTLA-4 (e.g. ipilimumab), PD-1 (e.g. pembrolizumab) and PD-L1 (atezolizumab) inhibitors (see mechanisms below!). While ICIs have advanced the treatment of multiple cancers, including melanoma, the side effects are the real deal and can manifest in the skin. This retrospective cohort study evaluated a national claims database with 8,637 cancer patients treated with ICIs in order to better understand cutaneous immune-related adverse events. They found that approximately 25% of patients treated with ICIs developed cutaneous side effects. The most common side effects included pruritus, mucositis, erythroderma, maculopapular eruption, Grover disease, and autoimmune/inflammatory type skin diseases such as vitiligo, lichen planus, and bullous pemphigoid (makes sense since your immune system is being revved up!). Median time to onset was 113 days (3-4 months) into treatment. Limitations include the retrospective nature of this study and inability to chart review to determine dermatologic diagnoses captured by broader diagnostic codes. Regardless, this type of study can help dermatologists better understand the timing and types of side effects to expect from ICIs and better diagnose and manage these patients.
|
Abbreviation help:
anticytotoxic T-lymphocyte antigen-4= CTLA-4 anti-programmed cell death protein-1 = PD-1 anti-programmed cell death ligand-1= PD-L1 Mechanism of CTLA-4 inhibitors: CTLA-4 typically binds CD80/86 on antigen presenting cells and inactivates T cells. CTLA-4 inhibitors bind CTLA-4 on T cell, thus preventing inactivation of T cell |
Mechanism of PD-1 and PD-L1 inhibitors (this can show up on boards!):
PD-L1 on tumor cell binds PD-1 on T cell to prevent T cell killing of tumor cell Anti-PD-L1 binds PD-L1 on tumor cell which allows T cell killing of tumor cell Anti-PD-1 binds PD-1 on T cell which allows T cell killing of tumor cell |
Rosacea: a future a little less rosy
Journal of Investigative Dermatology
Fun fact: the word rosacea stems from the latin word rosaceus, which means “made of roses.” While we do not completely understand why some people have rosacea, it is known that pituitary adenylate cyclase activating protein (PACAP) is up-regulated in these patients. A double-blind, randomized, placebo-controlled study (n=35) further investigated the specific role PACAP38 plays in patients with rosacea, and whether or not sumatriptan can subsequently alleviate symptoms. After intravenous infusion with PACAP38, patients experienced a 90% increase in blood flow to facial skin, with a 56% increase in dilation to the superficial temporal artery. Those in the treatment arm who received a second injection with sumatriptan experienced a subsequent reduction in blood flow to the skin for 50 minutes (p=0.023), reduced facial flushing (p=0.001), and reduced facial edema (p=<0.001). These results are significant because they not only highlighted how PACAP38 can induce rosacea, but also showed that sumatriptan can be used to alleviate symptoms. This may mean that rosacea patients’ futures could be a little less rosy.
Journal of Investigative Dermatology
Fun fact: the word rosacea stems from the latin word rosaceus, which means “made of roses.” While we do not completely understand why some people have rosacea, it is known that pituitary adenylate cyclase activating protein (PACAP) is up-regulated in these patients. A double-blind, randomized, placebo-controlled study (n=35) further investigated the specific role PACAP38 plays in patients with rosacea, and whether or not sumatriptan can subsequently alleviate symptoms. After intravenous infusion with PACAP38, patients experienced a 90% increase in blood flow to facial skin, with a 56% increase in dilation to the superficial temporal artery. Those in the treatment arm who received a second injection with sumatriptan experienced a subsequent reduction in blood flow to the skin for 50 minutes (p=0.023), reduced facial flushing (p=0.001), and reduced facial edema (p=<0.001). These results are significant because they not only highlighted how PACAP38 can induce rosacea, but also showed that sumatriptan can be used to alleviate symptoms. This may mean that rosacea patients’ futures could be a little less rosy.
QUESTION OF THE WEEK
NEJM CHALLENGE QUESTION
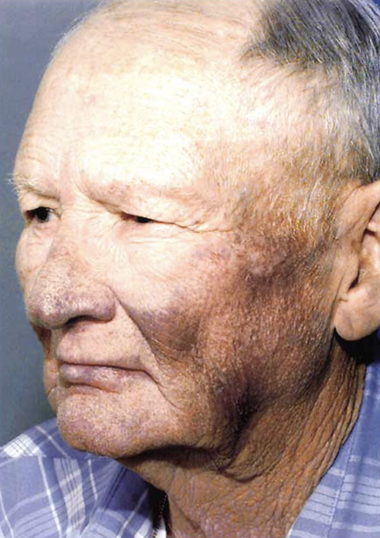
This 61-year-old man is receiving care for an arrhythmia. What is the cause of his appearance?
Answer:
Amiodarone
Patient had a defibrillator implanted after cardiac arrest in 1989 and treated with amiodarone for five years. During therapy, he developed the blue-gray appearance is a result of treatment with amiodarone
Amiodarone causes slate gray hyperpigmentation as seen in this photo. It is a phototoxic drug reaction and the blue-gray hyperpigmentation is usually in sun exposed areas. TCAs and diltiazem can also cause slate gray hyperpigmentation in sun exposed areas.
Procainamide- high risk for drug-induced systemic lupus erythematosus but not blue-gray discoloration
Bretylium - does not result in discoloration of skin
Sotalol - does not result in discoloration of skin
Hydralazine - high risk for drug-induced systemic lupus erythematosus but not blue-gray discoloration
Other drug- induced pigmentary changes:
- Procainamide
- Bretylium
- Amiodarone
- Sotalol
- Hydralazine
Answer:
Amiodarone
Patient had a defibrillator implanted after cardiac arrest in 1989 and treated with amiodarone for five years. During therapy, he developed the blue-gray appearance is a result of treatment with amiodarone
Amiodarone causes slate gray hyperpigmentation as seen in this photo. It is a phototoxic drug reaction and the blue-gray hyperpigmentation is usually in sun exposed areas. TCAs and diltiazem can also cause slate gray hyperpigmentation in sun exposed areas.
Procainamide- high risk for drug-induced systemic lupus erythematosus but not blue-gray discoloration
Bretylium - does not result in discoloration of skin
Sotalol - does not result in discoloration of skin
Hydralazine - high risk for drug-induced systemic lupus erythematosus but not blue-gray discoloration
Other drug- induced pigmentary changes:
- Hyperpigmentation= minocycline, chemotherapeutics, zidovudine also known as azidothymidine (AZT), antimalarials (e.g. hydroxychloroquine), and heavy metals
- Hypopigmentation= tyrosine kinase inhibitors (imatinib), phenols/catechols (e.g. hydroquinone), sulfhydryls (methimazole), corticosteroids, azelaic acid