TWENTY-EIGTH ISSUE
FEBRUARY 15, 2023
Risk of Infection in Children With Psoriasis Receiving Treatment With Ustekinumab, Etanercept, or Methotrexate Before and After Labeling Expansion
JAMA Dermatology
Ustekinumab is stellar(a) at keeping infection risk low!
While systemic agents are being used with increasing frequency in the treatment of pediatric psoriasis patients, the risk of serious infection with their use is not well characterized. This cohort study utilized insurance claims data from 2,338 patients over a 12-year period to estimate 6-month rate of infections among pediatric patients who were initiated on new treatment with ustekinumab, etanercept, or methotrexate. Propensity-score adjusted incidence rates were determined and reported per 1000 person-years.
What did they find?
- Incidence rate of inpatient serious infection was 18.4 (3 events) for ustekinumab, 25.6 (9 events) for etanercept, and 14.9 (8 events) for methotrexate users per 1000 person-years
- Incidence rate of outpatient infection requiring treatment was 254.9 (39 events) for ustekinumab, 435.7 (139 events) for etanercept, and 433.6 (209 events) for methotrexate users per 1000 person-years
- Adjusted rate ratio of outpatient infections was 0.58 (95% CI, 0.41-0.83) for ustekinumab vs etanercept, 0.66 (95% CI, 0.48-0.91) for ustekinumab vs methotrexate, and 0.95 (95% CI, 0.75-1.21) for etanercept vs methotrexate
Main Takeaways: Overall, incidence of infection with systemic treatments was low. Compared with methotrexate and etanercept, ustekinumab was not associated with an increased risk of infections among this cohort of pediatric patients with psoriasis.
Tumor necrosis factor inhibitors sounding scary? Not to worry!
Tumor necrosis factor inhibitors (TNFis) and methotrexate (MTX) are immune-modulating agents used to treat a wide variety of autoimmune conditions, including psoriasis, psoriatic arthritis, and hidradenitis suppurativa. Researchers wondered whether exposure to these medications could worsen severity of Covid-19 infection and lead to increased rates of hospitalization or Covid-related mortality.
In this retrospective cohort study, researchers identified patients in the TriNetX database diagnosed with Covid-19 who had been treated with TNFis, MTX, or both within 1 year of infection. The primary outcome was hospitalization or death within 45 days of Covid-19 diagnosis.
What did they find?
Main Takeaway: Patients treated with tumor necrosis factor inhibitors, methotrexate, or both did not appear to experience higher rates of hospitalization or mortality after Covid-19 diagnosis compared to control patients.
Tumor necrosis factor inhibitors (TNFis) and methotrexate (MTX) are immune-modulating agents used to treat a wide variety of autoimmune conditions, including psoriasis, psoriatic arthritis, and hidradenitis suppurativa. Researchers wondered whether exposure to these medications could worsen severity of Covid-19 infection and lead to increased rates of hospitalization or Covid-related mortality.
In this retrospective cohort study, researchers identified patients in the TriNetX database diagnosed with Covid-19 who had been treated with TNFis, MTX, or both within 1 year of infection. The primary outcome was hospitalization or death within 45 days of Covid-19 diagnosis.
What did they find?
- Patients who had been exposed to TNFis, MTX, or both prior to Covid-19 diagnosis were significantly less likely to have been hospitalized, with a risk difference of 2.786% in patients treated with both TNFis and MTX (risk ratio [RR] = 0.860 [95% confidence interval {CI} 0.828, 0.893]
- There was a risk difference of 2.933% in the TNFis only group (risk ratio = 0.841 [95% CI 0.781, 0.907]), and 2.952% in the MTX only group (RR = 0.863 [95% CI 0.821, 0.908)
- There was no significant risk difference for mortality (0.087% for TNFis and MTX, 0.385% for TNFis only, and 0.016% for MTX only (RR = 0.961 [95% CI 0.854, 1.083], RR = 0.776 [95% CI 0.59, 1.02], RR = 1.006 [95% CI 0.866, 1.168])
Main Takeaway: Patients treated with tumor necrosis factor inhibitors, methotrexate, or both did not appear to experience higher rates of hospitalization or mortality after Covid-19 diagnosis compared to control patients.
Efficacy and safety of topical brepocitinib for the treatment of mild-to-moderate atopic dermatitis: a phase IIb, randomized, double-blind, vehicle-controlled, dose-ranging and parallel-group study
British Journal of Dermatology
I scream for skin creams!
Atopic dermatitis (AD) is an extremely itchy, inflammatory skin condition. Targeting inflammatory pathways, including the JAK pathway, has been implicated in treatment of AD. These include topical medications (e.g. delgocitinib) and systemic medications (e.g. ruxolitinib). The authors performed a phase IIb, multicenter, randomized, double-blind, vehicle controlled, parallel-group, dose ranging study to examine the safety and efficacy of topical brepocitinib, a small molecule TYK2/JAK1 inhibitor, which is thought to act on the IL-12 and IL-23 pathways, on the management of AD.
292 patients with mild to moderate AD were randomized to 8 treatment arms for 6 weeks: vehicle, 0.15 brepocitinib, 0.3%, 1.0% or 3.0% cream either once daily or twice daily. Endpoints included the percent change in EASI total score from baseline at week 6 and the proportion of patients achieving IGA scores of 0, 1, or a reduction of 2 or more points from baseline at week 6.
What did they find?
Main Takeaways: Topical brepocitinib is well-tolerated and effective for treatment of AD.
Atopic dermatitis (AD) is an extremely itchy, inflammatory skin condition. Targeting inflammatory pathways, including the JAK pathway, has been implicated in treatment of AD. These include topical medications (e.g. delgocitinib) and systemic medications (e.g. ruxolitinib). The authors performed a phase IIb, multicenter, randomized, double-blind, vehicle controlled, parallel-group, dose ranging study to examine the safety and efficacy of topical brepocitinib, a small molecule TYK2/JAK1 inhibitor, which is thought to act on the IL-12 and IL-23 pathways, on the management of AD.
292 patients with mild to moderate AD were randomized to 8 treatment arms for 6 weeks: vehicle, 0.15 brepocitinib, 0.3%, 1.0% or 3.0% cream either once daily or twice daily. Endpoints included the percent change in EASI total score from baseline at week 6 and the proportion of patients achieving IGA scores of 0, 1, or a reduction of 2 or more points from baseline at week 6.
What did they find?
- There were no serious adverse effects or deaths
- There were no dose-dependent trends in adverse effects related to brepocitinib
- 1% brepocitinib once daily and twice daily achieved significantly greater reductions in EASI score at week 6. Other doses trended in the same direction but did not reach significance compared to vehicle. This is likely due to the relatively small sample sizes for each treatment arm, and larger studies are required to further investigate the effect sizes.
- In 5 out of 6 active treatment arms, proportions of patients reaching IGA endpoints of 0, 1, or a reduction of greater than 2 from baseline were significantly increased
Main Takeaways: Topical brepocitinib is well-tolerated and effective for treatment of AD.
Are mucocutaneous symptoms associated with severity of MIS-C cases?
Journal of Pediatric Dermatology
Don’t “misc” mucocutaneous symptoms in MIS-C!
Multisystem inflammatory syndrome in children (MIS-C) is a rare condition associated with SARS-CoV-2, that usually occurs 2-6 weeks after COVID-19 infection. MIS-C commonly presents with fever, diarrhea and vomiting, and mucocutaneous symptoms such as rash, conjunctivitis, periorbital edema, and palmoplantar erythema. In this study, the researchers conducted a retrospective cohort study of 66 patients under the age of 21 hospitalized for suspected MIS-C to investigate the relationship between mucocutaneous symptoms and MIS-C severity in different age and racial groups.
What did they find?
- Median age of children with mucocutaneous symptoms was 9.8 years (vs 11.4 years)
- 56 patients exhibited mucocutaneous findings on admission: rash (n=42), conjunctivitis (n=39), cracked lips (n=21), and sore throat (n=15)
- Children under age 5 were more likely to have red lips (33.3% vs 6.3%, p = 0.01)
- Children aged 5-21 were more likely to experience sore throats (31.3% vs 0%, p = 0.01)
- Severe MIS-C was defined as patients with MIS-C admitted to the PICU
- Older children aged 5-21 with mucocutaneous symptoms had higher odds of severe MIS-C compared to children under age 5 (OR 5.43, p = 0.02)
- Black children with mucocutaneous symptoms had higher odds of MIS-C compared to white children (OR 3.30, p = 0.047)
Main Takeaways: This study found that older children and black children with mucocutaneous symptoms are more likely to develop severe MIS-C.
innovations in dermatology
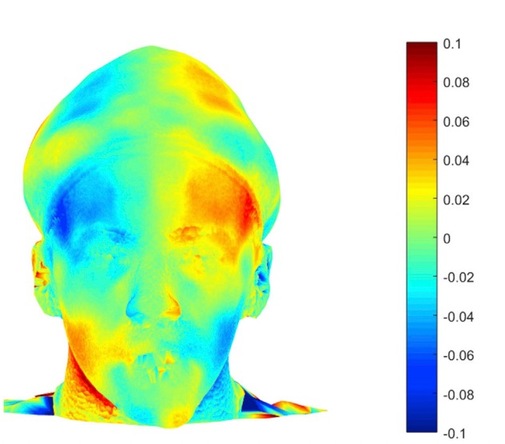
So, you’re telling me I’m not symmetric?
Facial morphea is an autoimmune mediated disease that causes sclerosis and inflammation of the skin and tissue, which can lead to asymmetry and craniofacial defects. Currently, serial photography, MRI, and clinical scoring are used when evaluating disease progression. 3D stereophotogrammetry presents a non-invasive, radiation-free modality that uses non-metric cameras and mathematical computation to produce a rendering of the facial features of the patient. Use of this technology may provide patients with better clinical outcomes and improve quality of life.
What did they find?
Facial morphea is an autoimmune mediated disease that causes sclerosis and inflammation of the skin and tissue, which can lead to asymmetry and craniofacial defects. Currently, serial photography, MRI, and clinical scoring are used when evaluating disease progression. 3D stereophotogrammetry presents a non-invasive, radiation-free modality that uses non-metric cameras and mathematical computation to produce a rendering of the facial features of the patient. Use of this technology may provide patients with better clinical outcomes and improve quality of life.
What did they find?
- Twenty-three participants with a diagnosis of facial linear morphea had 3D stereolithography for asymmetry analysis with >1.2 mm mathematical difference of asymmetry considered pathologic
- Fourteen patients (61%) were detected to have pathologic asymmetry in more facial regions than what was documented in clinical examination
- Twenty patients showed pathological asymmetry in at least one facial region
- Experts rated 5 patients to have a greater rating of asymmetry when examined with 3D stereophotogrammetry compared to traditional photographs
- PGA-D scores were significantly correlated to the 3D stereophotogrammetry for mouth asymmetry (p=.0021) and cheek asymmetry (p=0.04), however the LoSDI score did not show a significant correlation
Fig. 1. 3D stereophotogrammetry in detection of facial asymmetry in a 15 year old boy with craniofacial morphea.
Main Takeaway: 3D stereophotogrammetry proves to be a reliable detection tool with greater sensitivity than the current methods. This tool may be useful alongside physical exam in monitoring the disease progression of craniofacial morphea.